One stone, two birds: silica nanospheres significantly increase photocatalytic activity and colloidal stability of photocatalysts
Kowsalya D. Rasamani, Jonathan J. Foley IV, Yugang Sun
Nano Futures
2018
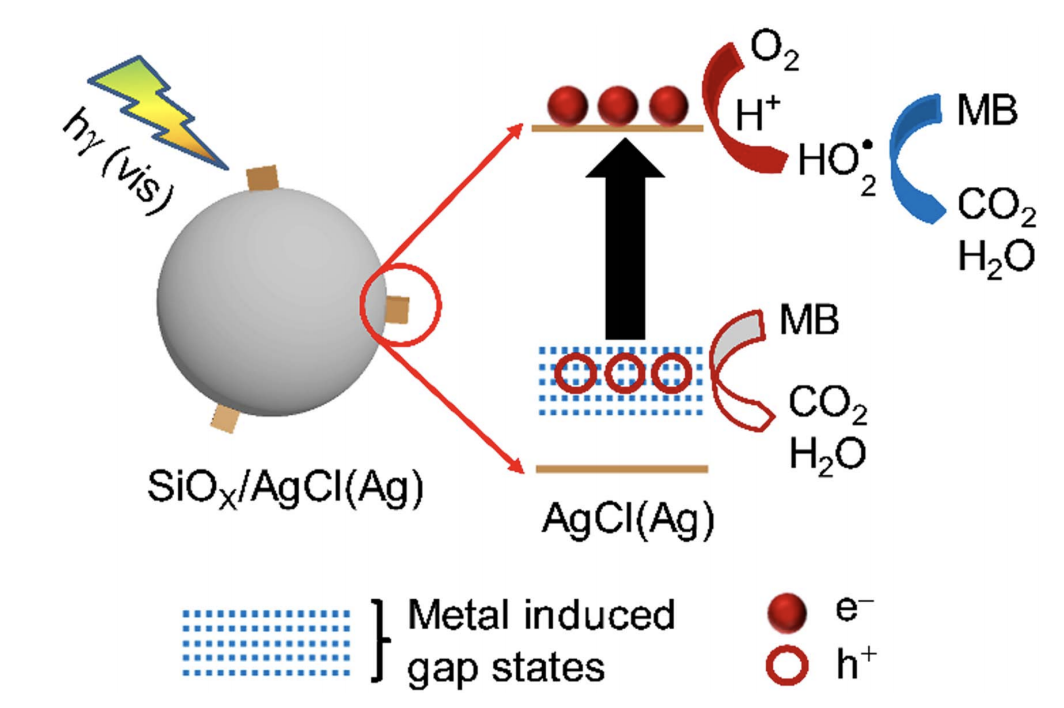
Silver-doped silver chloride [AgCl(Ag)] nanoparticles represent a unique class of visible-light-driven photocatalysts, in which the silver dopants introduce electron-abundant mid-gap energy levels to lower the bandgap of AgCl. However,free-standing AgCl(Ag) nanoparticles, particularly those with small sizes and large surface areas, exhibit low colloidal stability and low compositional stability upon exposure to light irradiation, leading to easy aggregation and conversion tometallic silver and thus a loss of photocatalytic activity. These problems could be eliminated by attaching the small AgCl(Ag) nanoparticles to the surfaces of spherical dielectric silica particles with submicrometer sizes. The high optical transparency in the visible spectral region (400–800 nm), colloidal stability, and chemical/electronic inertness displayed by the silica spheres make them idealfor supporting photocatalysts and significantly improving their stability. The spherical morphology of the dielectric silica particles can support light scattering resonances to generate significantly enhanced electric fields near the silica particle surfaces, on which the optical absorption cross-section of the AgCl(Ag) nanoparticles is dramatically increased to promote their photocatalytic activity. The hybrid silica/AgCl(Ag)structures exhibit superior photocatalytic activity and stability, suitable for supporting photocatalysis sustainably;for instance, their efficiency in the photocatalytic decomposition of methylene blue decreases by only ∼9% even after ten cycles of operation.