Cage versus Prism: Electronic Energies of the Water Hexamer
Jonathan J. Foley IV and David A. Mazziotti
J. Phys. Chem. A
2013
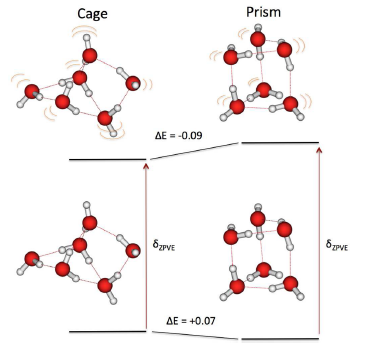
Recent experiments show that the cage isomer of the water hexamer is lower in energy than the prism isomer near 0 K, and yet state-of-the-art electronic structure calculations predict the prism to be lower in energy than the cage at 0 K. Here, we study the relative energies of the water hexamers from the parametric two-electron reduced density matrix (2-RDM) method in which the 2-RDM rather than the wave function is the basic variable of the calculations. In agreement with experiment and in contrast with traditional wave function methods, the 2-RDM calculations predict the cage to be more stable than the prism after vibrational zero-point correction. Multiple configurations from the hydrogen bonding are captured by the method. More generally, the results are consistent with our previous 2-RDM applications in that they reveal how multireference correlation in molecular systems is important for resolving small energy differences from hydrogen bonding as well as other types of intermolecular forces, even in systems that are not conventionally considered strongly correlated.